Fish oil – 100 ml-e JEKOSIN
Fish oil – 100 ml-e JEKOSIN
DIETETIC SUPPLEMENT – ORAL DROPS
Bottle with 100 ml of fish oil for oral use.
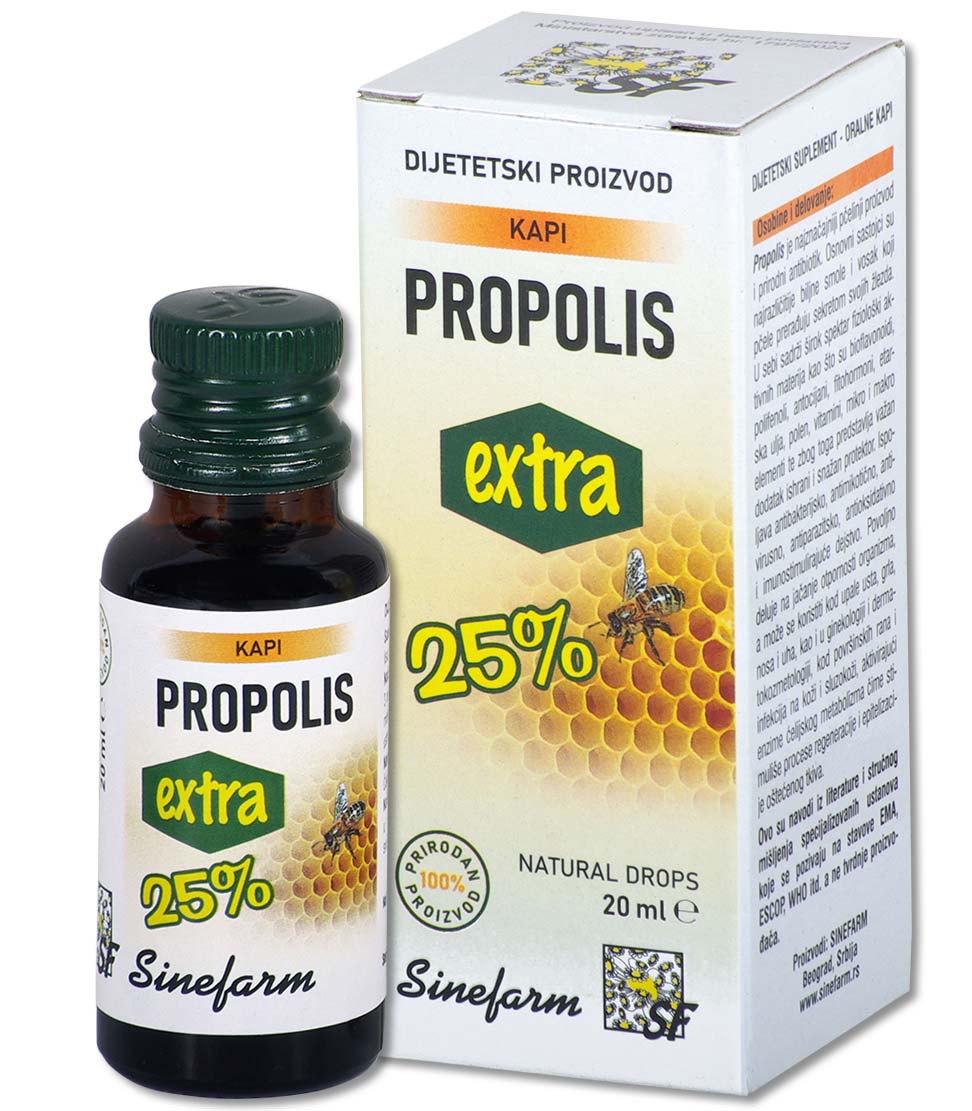
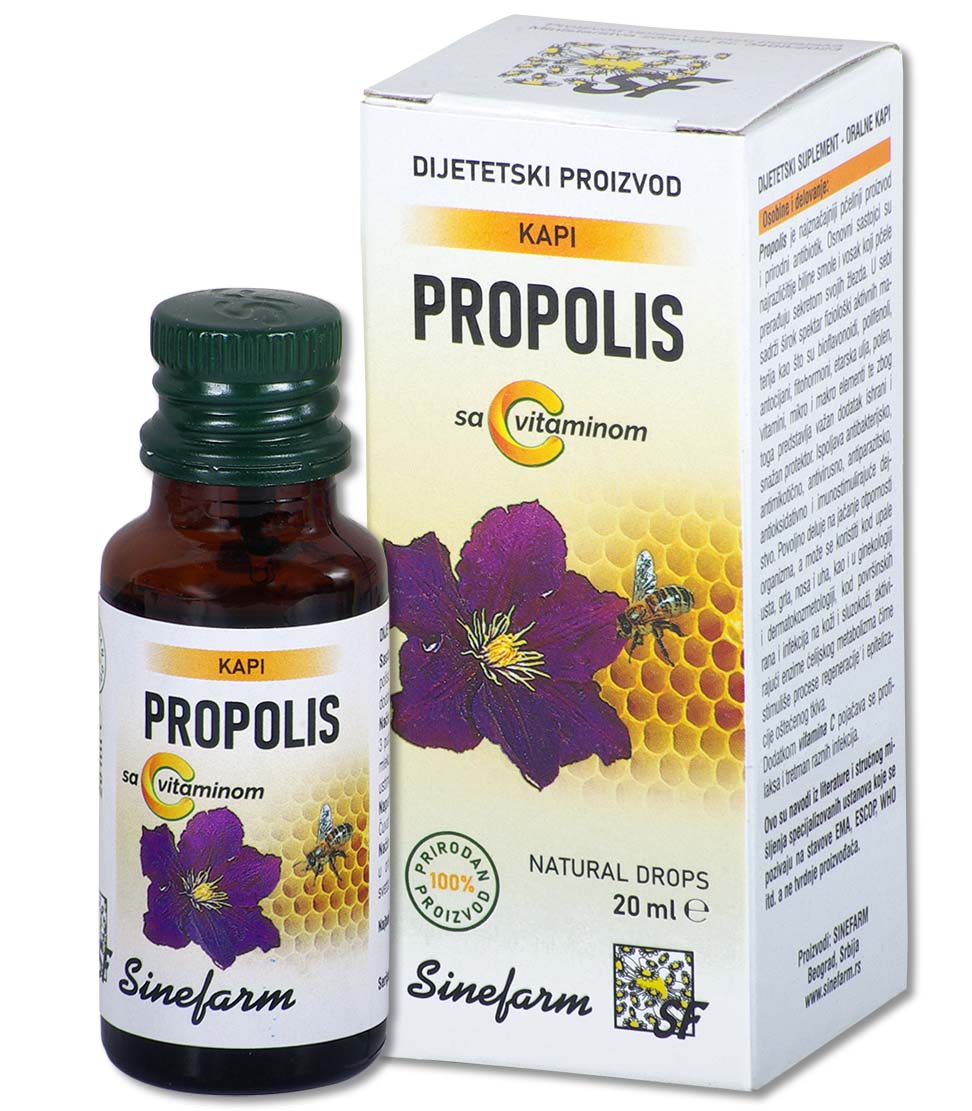
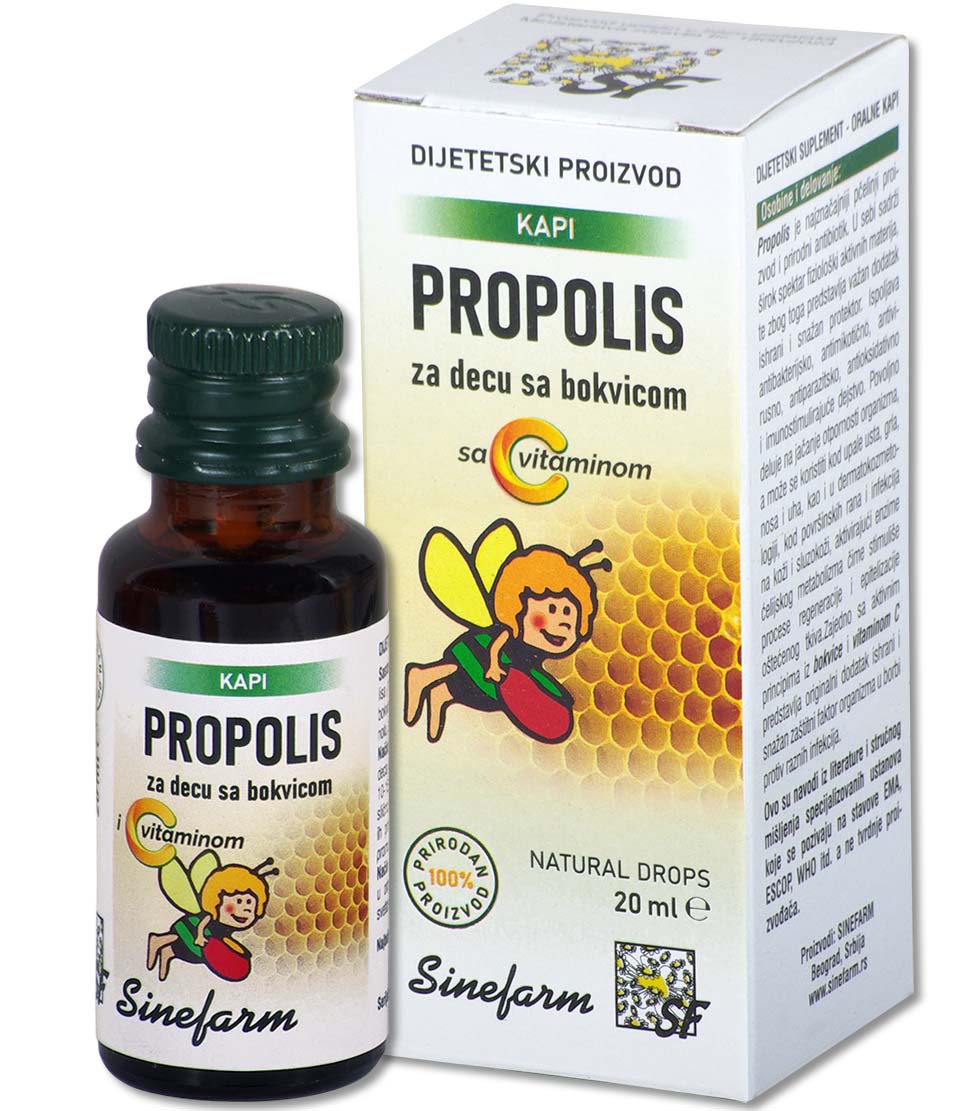
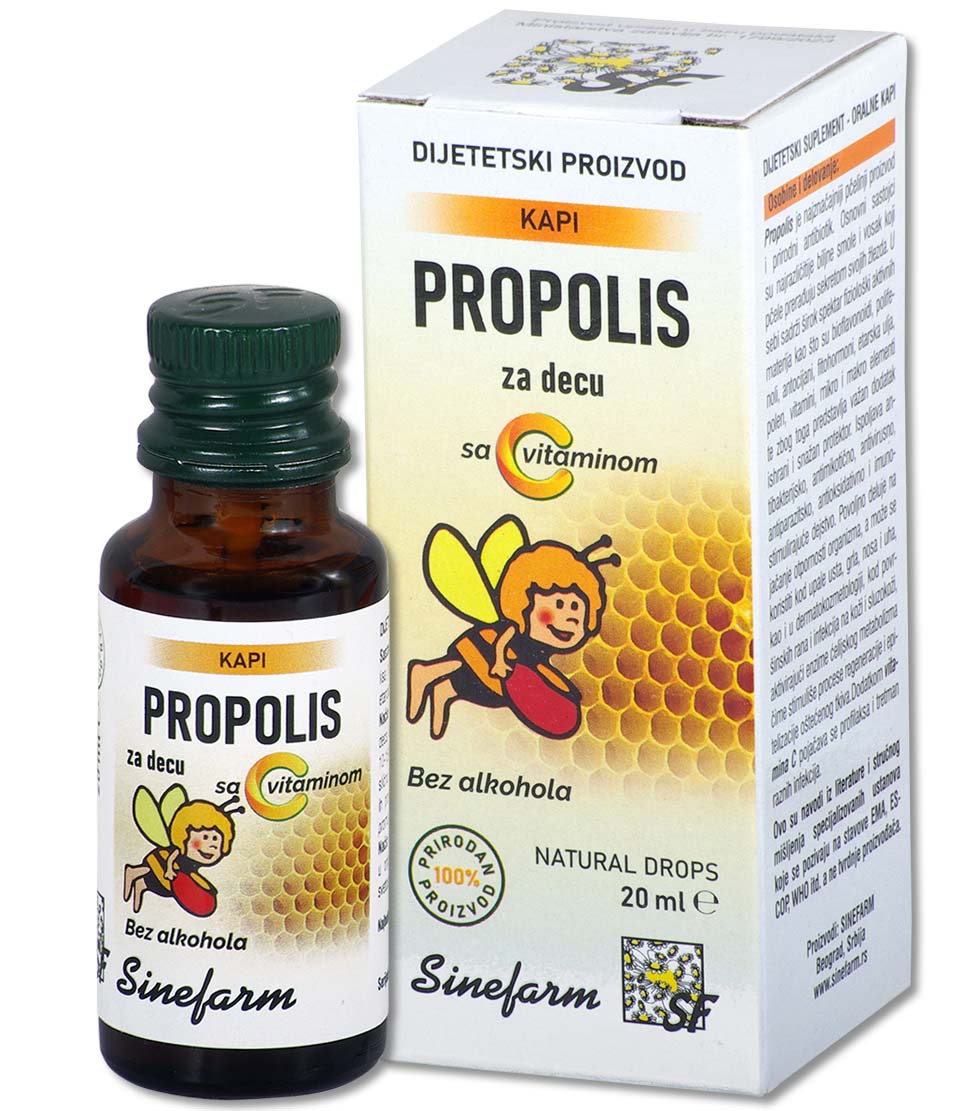
Characteristics and effects: Fish oil is obtained from the livers of North Sea fish.
It is a natural and rich source of vitamins A and D, and helps the utilization of calcium and phosphorus from the digestive tract and thus regulates the proper growth and development of the musculoskeletal system, so it is used in the treatment and prevention of rickets and osteomalacia in adults.
As a dietary supplement, especially in the winter months as a prevention, it is recommended for malnourished children, convalescents, etc.
It has a beneficial effect on the function of visual adaptation and increases the body’s resistance to infections.
These are excerpts from the literature and expert opinions of specialized institutions that refer to the positions of the EMA, ESCOP, WHO, etc. and not the manufacturer’s claims.
Ingredients: Fish oil
Energy value 3634 kJ/895 kcal per 100 ml.
| Active ingredients | In 2.5 ml oil (1/2 teaspoon) |
% NRV* (2,5 ml) |
In 100,0 ml oil |
| – vitamin A
– vitamin D |
878,4 µg RE
7,341 µg |
109,8
146,8 |
35,1 mg
293,6 µg |
* NRV (Nutritional Reference Value)
Instructions for use: For these conditions, adults and children over 4 years of age should take half a teaspoon once a day.
N.B. Do not exceed the recommended daily dose.
Supplements may not be used as a substitute for a varied diet.
Keep out of the reach of children.
Warning: The drops should not be used by anyone who is hypersensitive to any ingredient.
Not recommended for pregnant or breastfeeding women; for children under 4 years of age consult a pediatrician.
Caution is required in diabetics, people on anticoagulant therapy and those preparing for surgery. Also with long-term use of non-steroidal antirheumatic drugs, and people suffering from haemophilia and diabetes. Consult your physician.
How to store: Keep closed in original packaging, protected from light and moisture, at a temperature of up to 25° C.
Product entered in the database of the Ministry of Health No 1781/2018 of 29.08.2018.